
Artos
Best In Show - New Product Competition

Information
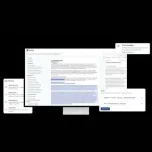
Artos is an AI-powered document authoring platform purpose-built for the life sciences industry. Backed by Y Combinator, Artos helps pharmaceutical and biotech companies streamline the creation of complex regulatory and R&D documents—such as INDs, NDAs, BLAs, protocols, investigator brochures, and clinical or CMC study reports. By combining agentic AI systems with automation, traceability, and support for varied data formats, Artos dramatically improves both the speed and accuracy of scientific documentation.
Unlike generic AI tools, Artos is specifically engineered to meet the transparency, reliability, and reproducibility standards required in pharmaceutical R&D. Its modular architecture scales across the diverse and often messy workflows of drug development, allowing teams to realize over 50% time or cost savings within the first year of use.
Key capabilities include full transparency into data processing, automated workflows for dynamic document updates, and seamless handling of unstructured input files (including PDFs, Excel sheets, and images). Artos eliminates the need for prompt engineering by using intelligent agents trained to produce reliable, reproducible outputs. Users can easily integrate company-specific templates and style guides without manual formatting, while built-in inconsistency intelligence flags discrepancies across documents.
With Artos, life sciences teams can focus on science and strategy rather than formatting and busywork. The platform accelerates timelines, reduces risk, and enables scalable, compliant document authoring from discovery through approval. Learn more at www.artosai.com
Product Link
Link to Vote
